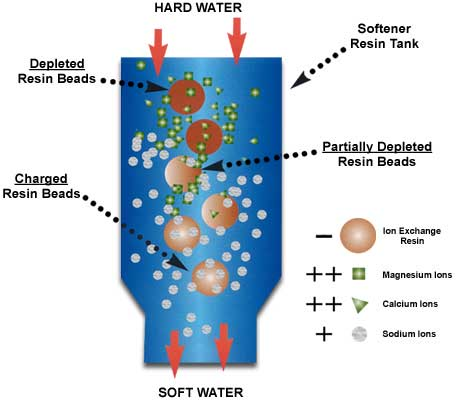
Principles of Zeolite Softening Sodium zeolite softeners use exchange resins made of polystyrene. These resins have sodium ions loosely attached and will readily give up sodium for more desirable ions such as calcium and magnesium. This exchange is only for cations (positively charged ions). This is why sodium zeolite resin is referred to as a cation […]